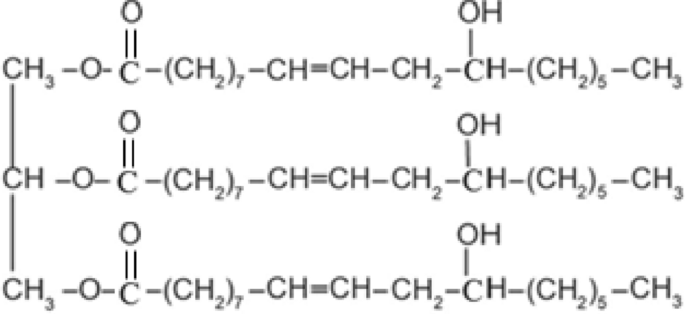
Tentative mechanism
It is anticipated that addition of APS aqueous solution to a thermostated chemical reaction containing a mixture of polyethylene glycol and castor oil present in plenty compared to PEG would cause the following reactions27,28,29:
-
(a)
Generation of free radicals
$${\text{S}}_{2} {\text{O}}_{8}^{2 – } \to 2{\text{SO}}_{4}^{ – \cdot }$$
(1)
$${\text{SO}}_{4}^{ – \cdot } + {\text{H}}_{2} {\text{O}} \to {\text{HSO}}_{4}^{ – } + {}^{ \cdot }{\text{OH}}$$
(2)
$${\text{PEG-OH}} + {\text{R}}^{ \cdot } \to \mathop {{\text{PEG-O}}^{ \cdot } }\limits_{{({\text{PEG}}\;{\text{macroradicals}})}} + {\text{RH}}$$
(3)
where R⋅ is the free radicals species (SO⋅4− and/or HO⋅) generated by the decomposition of ammonium per sulfate in aqueous medium.
-
(b)
Reaction of castor oil via double bonds28,29:

(4)

(5)
-
(c)
Reaction of castor oil via hydroxyl groups27,29:

(6)
-
(d)
Reaction of castor oil via both the double bonds and hydroxyl groups27,29:

(7)
Thus, the aforementioned reaction medium includes a mixture of un-reacted castor oil, castor oil grafted with PEG species and/or sulfate groups, as well as different oxidized species; all in state of entanglement. However, the grafted castor oil acts as a self-emulsifier for the remaining unreacted castor oil since castor oil is present in the reaction medium in plenty with respect to PEG. For simplicity, this mixture will be referred as CAO/PEG hybrid. The major factors affecting CAO/PEG hybrid formation will be studied. Results obtained along with appropriate discussion follow.
Factors affecting CAO/PEG hybrid formation
Initiator concentration
Table 1 shows the effect of APS/PEG weight ratio on CAO/PEG hybrid emulsion state. It is clear that, increasing APS/PEG weight ratio to 15% is accompanied by a gradual improvement in the hybrid emulsion state which could associated with increasing of the free radicals number initiating the formation of CAO-g-PEG species as an emulsifier responsible for emulsification of the remaining unreacted CAO, since the PEG/CAO weight ratio is 35%. The further increasing in that ratio, within the range studied, has no effect on the emulsion state, suggesting the rapid rate of termination due to increasing of the APS concentration1,2,28.
Reaction temperature
Table 2 illustrates the impact of reaction temperature on the CAO/PEG hybrid emulsion state. As is evident, raising the reaction temperature from 60 to 80 °C significantly improves hybrid emulsion state, the point that reflects the temperature favorable effect on the initiate or decomposition, i.e. increasing of the free radicals species, as well as increasing of the reactants molecules mobility, the matter that certainly enhances formation of the CAO-g-PEG species as emulsifier and consequently state of the hybrid emulsion1,2,28. Moreover, the further increase in reaction temperature, i.e. beyond 80 and up to 90 °C, has no effect on the hybrid emulsion state. It seems that at higher temperatures, the high rate of initiation is outweighed by a faster rate of termination1,2,28.
Reaction time
Table 3 shows the CAO/PEG hybrid emulsion stateas a functionin the reaction time. It is obvious that increasing of the reaction time from 15 to 60 results in a gradual improvement in the hybrids emulsion state as a result for increasing of APS extent of decomposition that in turn enhances the hybrid formation as well as upgrades its emulsion state. Prolonging the reaction time does not affect state of the hybrid emulsion which can be interpreted in terms of the depletion in the PEG amount and/or APS concentration30,31,32.
PEG/CAO weight ratio
Table 4 depicts the effect of the PEG/CAO weight ratio on emulsion state of the formed hybrid. It is well seen that increasing of that ratio from 0 to 35% in the reaction medium gives rise to a progressive promotion in the hybrid emulsion state. This may be a direct consequence for increasing of the CAO-g-PEG species formation in the reaction medium in addition to the role of the unreacted PEG in dispersing of the formed hybrids2,3,28,33. The further increasing in that ratio, i.e. at 45%, does not affect the hybrid emulsion state.
PEG molecular weight
Table 5 shows the effect of PEG molecular weight on emulsion state of the prepared hybrids. It is clear that increasing of PEG molecular weight from 300 to 1000 Da is accompanied with a gradual promotion in emulsion state of the formed hybrid. Moreover, using of the PEG of the molecular weight 2000 Da in preparation of the CAO/PEG hybrids keeps stability of the hybrid emulsion. Unfortunately, PEG 4000 impairs state of the formed hybrid emulsion which may be related to increasing of the reaction medium viscosity that hinders the molecular collisions of the reactants and/or decreasing of the hydroxyl groups number by increasing the PEG molecular weight, the matter that indeed reduces the extent of formation of CAO-g-PEG species3,33.
Inclusion of the CAO/PEG hybrids emulsionsin easy-care finishing of cotton fabric
The performance properties of the CAO/PEG hybrids emulsions treated fabric
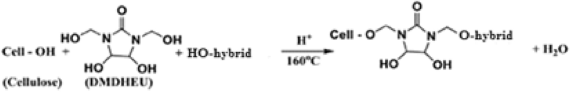
Table 6 shows the effect of incorporating of CAO/PEG1000 or CAO/PEG2000 hybrid emulsion in the easy care finishing formulations of cotton fabric on performance properties of that fabric. It is obvious that inclusion of either of such hybrids emulsions in the finishing bath leads to an enhancement in nitrogen content, wrinkle recovery angle, stiffness, and softness along with a reduction in tensile strength, whiteness index, and wettability properties of treated fabric, compared to the untreated fabric sample. The matter that can be associated with the fixation of such hybrids ingredients onto/within the treated fabric structure3,21,22 as represented by Eq. (8). Moreover, during the reaction of binding of that hybrids ingredients to the cotton fabric (Eq. 8), other reactions may be also occurred that are crosslinking of the cotton cellulose (Eq. 9) and/or crosslinking of the hybrid ingredients (Eq. 10) forming a deposit onto/within the finished fabric resulting in extra softness effect to the finished fabric surface.
(8)
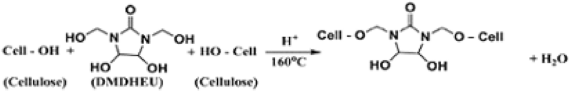
(9)
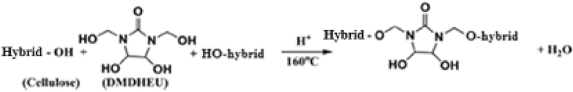
(10)
Table 6 illustrates also that replacing the CAO/PEG1000 with CAO/PEG2000 in the above mentioned finishing bath leads to an enhancement in extents of tensile strength, whiteness index, and stiffness along with a reduction in nitrogen content, resiliency, wettability and softness properties of the treated fabric. It seems that increasing of the PEG molecular weight from 1000 to 2000 Da renders the CAO/PEG2000 hybrid emulsion particles to be larger than that of the CAO/PEG2000 hybrid emulsion34,35,36,37,38 resulting in a rougher treated fabric surface38 as well as alteration in extents of the above mentioned properties.
The antibacterial properties of the CAO/PEG hybrids emulsions treated fabric
Finishing conditions: [Hybrids emulsions], 40 g/l; [DMDHEU], 60 g/l; [(NH4)2SO4], 4 g/l; [Ag-NPs], 2 g/l; wet pick up, 100%; drying, 100 °C/3 min; curing, 160 °C/3 min. G+ ve: St. aurous; G− ve: E. coli. Values in parentheses indicate retained antibacterial properties of treated fabric after 10 and 20 laundering cycles.
Table 7 shows the antibacterial properties of cotton fabric samples treated with different finishing formulations containing the aforementioned hybrids emulsions in absence or presence of silver nanoparticles (Ag-NPs). It is obvious that: (1) incorporating of such hybrids emulsions, regardless of the hybrid type, brings about an enhancement in the antibacterial activities of treated fabric against both the G− ve (E. coli) and G+ ve (S. auereus) bacteria reflecting the antibacterial properties of castor oil39,40, (2) the fabric sample treated with the CAO/PEG1000 hybrid emulsion has higher antibacterial activities than that treated with the CAO/PEG2000 hybrid emulsion; a point that reflects the antibacterial properties of PEG 100041, (3) inclusion of Ag-NPs in the easy care finishing bathes containing any of the above mentioned hybrids emulsions results in upgrading of the antibacterial properties of treated fabric. The matter that can be related to a generation of silver ions, in the presence of moisture, that attach to the bacterial DNA causing its inactivation according to Eq. (11) and/or generating of oxygen radicals that destroys the bacteria molecular structure according to Eq. (12)21, and (4) the antibacterial properties of both the hybrids emulsions treated fabric care durable for 20 washing cycles, suggesting the role of DMDHEU as a cross linker in binding of such hybrids ingredients to the cotton cellulose structure (Eq. 6).
$${\text{O}}_{{2({\text{aq}})}} + 4{\text{H}}_{3} {\text{O}}^{ + } + 4{\text{Ag}}_{{(s)}} \to 4{\text{Ag}}^{ + } _{{({\text{aq}})}} + 6{\text{H}}_{2} {\text{O}}$$
(11)
$${\text{H}}_{2} {\text{O}} + (1{\text{/}}2){\text{O}}_{2} \xrightarrow{{{\text{Ag}}^{ + } }}{\text{H}}_{2} {\text{O}}_{2} \to {\text{H}}_{2} {\text{O}} + ({\text{O}})$$
(12)
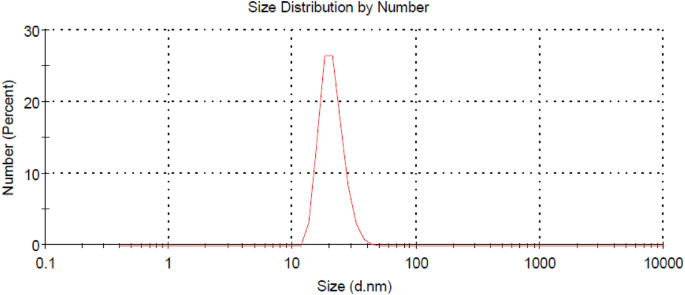
On the other hand, Fig. 2 shows the TEM image of the prepared Ag-NPs Whereas Fig. 3 shows the particle size distribution of such Ag-NPs. It is clear from Fig. 2 that size of the prepared Ag-NPs ranges from 15 to 27 nm whereas Fig. 3 confirms that the meandiameter of such particles is 21.04 nm.
Characterization of CAO/PEG1000 hybrid
Due to the performance as well as antibacterial properties of the CAO/PEG1000 hybrid emulsion treated fabric, it is selected to be characterized via FTIR and 1HNMRanalysis. Moreover, such hybrid emulsion particle size was examined via TEM analysis whereas its treated fabric was characterized via SEM and EDX analysis. In addition, Fig. 4 shows the visual appearance of the CAO/PEG1000 hybrid emulsion. On the other hand, the percent grafting of castor oil with PEG 1000 was calculated using the following equation24:
$$\% {\text{G}} = \left[ {\text{PEG grafted CAO}} \right]{/}\left[ {{\text{CAO}}} \right] \times {1}00\%$$
It was found that percent grafting was equal to 65.1% by considering weight of the PEG grafted castor oil equals 6.51 g and the original weight of castor oil is 10 g.
FTIR analysis
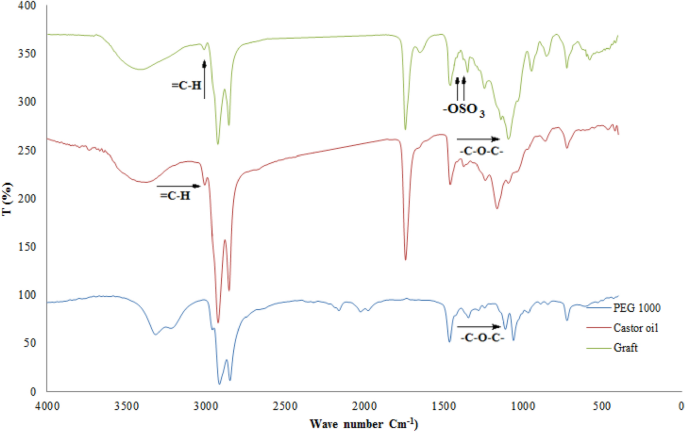
The FTIR spectra of castor oil, PEG 1000, and grafted castor oil are represented by Fig. 5. The spectrum of castor oil includes a peak at 3408 cm−1 corresponding to –OH stretching vibration, a peak at 2975 cm−1 corresponding to C–H (=C–H) stretching vibration, peaks at 2916 and 2848 cm−1 corresponding to CH2 asymmetrical and symmetrical respectively, a peak at 1734 cm−1 corresponding to C=O stretching of the ester group, a peak at 1624 cm−1 corresponding to the double bond of castor oil, and a peak at 941 cm−1 corresponding to –C=C– (trans) bending out of plane13,14,15,42. Moreover, the PEG 1000 spectrum includes peaks at 3301 cm−1 corresponding to –OH stretching, 2890 and 2840 cm−1 corresponding to aliphatic CH2 stretching vibration, 1452 cm−1 is due to binding vibration of CH2, and 1101 cm−1 corresponding to C–O–C stretching3.
On the other hand, the grafted castor oil spectrum includes some peaks resemble that of castor oil and PEG1000 such as a peak at 3401 cm−1 corresponding to –OH stretching of castor oil and PEG 1000, a peak at 3037 cm−1 corresponding to C–H (=C–H) stretching vibration of castor oil but with a lower intensity than that of the castor oil which may indicate opening of some double bonds of the castor oil during the hybrid formation, peaks at 2929 and 2858 cm−1 corresponding to aliphatic CH2 stretching vibration of castor oil and PEG 1000, a peak at 1745 cm−1 corresponding to C=O stretching of the ester group of castor oil, and a strong peak at 1101 cm−1 corresponding to –C–O–C– group but with a higher intensity than that of the PEG 1000 which confirms grafting of the castor oil viaits double bonds27,29. In addition, new peaks were appeared in the grafted castor oil spectrum at 1415 and 1380 cm−1 corresponding to sulfate groups which indicates replacement of some of –OH groups of castor oil with sulfate groups27,29,43.
1HNMR analysis
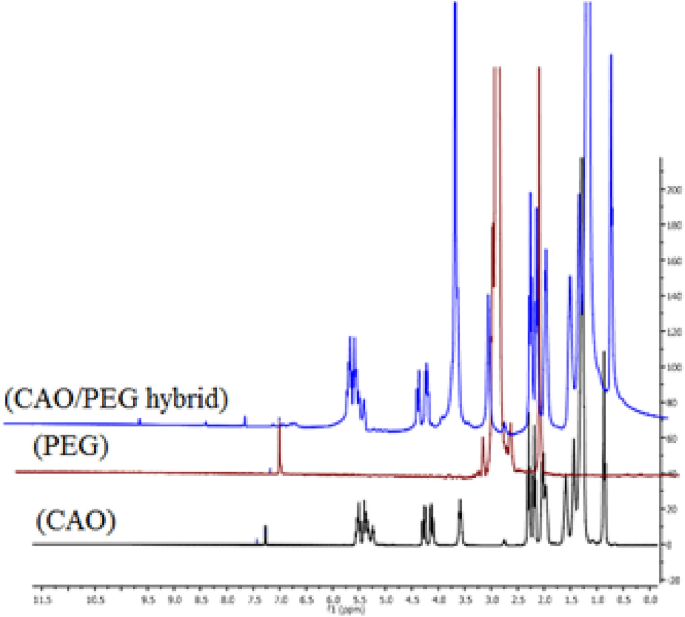
Figure 6 shows the 1H NMR of CAO/PEG1000 hybrid, castor oil and PEG1000. The following peaks were observed: 0.5–1 (s, Me), 1.0–1.5 (m, CH2), 1.75–2.0 (m, CH2 beside double bond), 2.0–2.5 (m, CH2 beside ketone and double bond), 3.0 (s, OH), 3.5 (s, OCH2), 4.0–4.5 (dd, olefinic-H), 5–5.5 (m, olefinic-H). By comparing the 1H NMR spectra of castor oil, polyethylene glycol, and the CAO/PEG1000 hybrid, it was noticed that there is an increasing in the integration of –CH2, –OCH2 and –OH groups indicating grafting of castor oil with polyethylene glycol.
TEM image
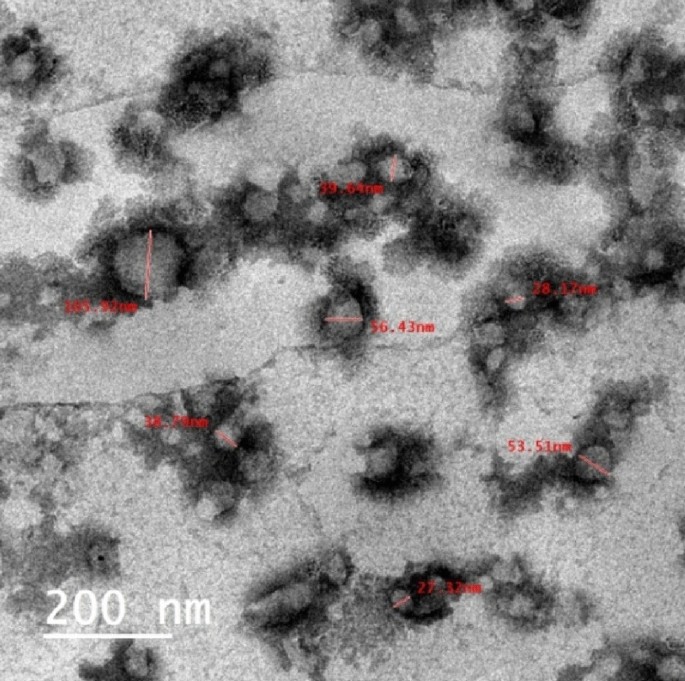
The particles size of the CAO/PEG1000 hybrid emulsion was characterized via TEM analysis and represented by Fig. 7. It is observed that such emulsion particles size is in the range of 27–105 nm.
SEM and EDX images
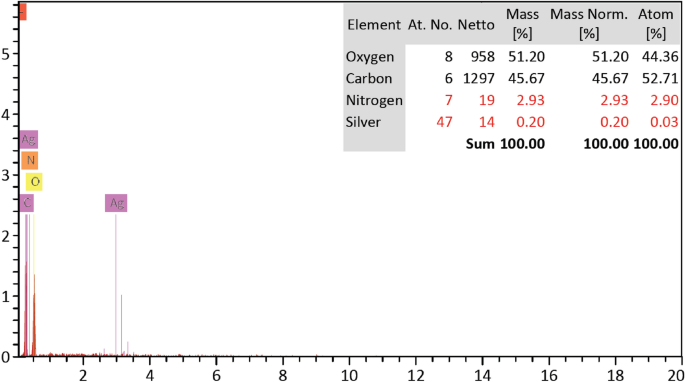
Figure 8 represents the SEM images of untreated cotton fabric and treated fabric with easy care finishing bath containing the prepared CAO/PEG1000 hybrid emulsion. It is well seen that the treated fabric surface has a deposited film. On the other hand, Fig. 9 shows the EDX analysis of the Ag-NPs loaded CAO/PEG1000 hybrid emulsion treated fabric which confirms the presence of Ag-NPs onto the finished fabric structure with Ag-content of 0.20% (w/w).
The wavelength, coloration and fastness properties of the dyed and dyed/finished cotton fabric
According to the higher performance as well as antibacterial properties of the CAO/PEG1000 hybrid emulsion, compared to the CAO/PEG2000 hybrid emulsion, cotton fabric samples were dyed with three reactive dyes and then treated with easy care finishing formulation containing that hybrid emulsion to illustrate the impact of that finishing on wavelength, coloration and fastness properties of the dyed samples. Given below the results along with an appropriate discussion.
The wavelength and coloration properties
The wavelength (λmax), color strength (K/S), as well as colorimetric data (L*, a*, b* and ∆E) magnitudes of the dyed cotton fabric samples before and after their treating with easy care finishing bath containing the CAO/PEG1000 hybrid emulsion are represented by Table 8. It is obvious that the un-finished dyed samples have different wavelengths, color strengths and colorimetric data reflecting the variation in functionality, molecular size, affinity, and mode of interaction of the nominated dyes44. Moreover, finishing of the dyed samples with the CAO/PEG1000 hybrid emulsion, regardless of reactive dye chemical structure, results in an increasing in K/S as well as a noticeable change in ∆E45 of the dyed/finished fabric samples, the matter that can be associated with the color-deepening effect of the CAO/PEG1000 hybrid emulsion as a textile softener21. Figure 10 shows the Reactive Yellow 2, Reactive Red 15, and Reactive Black 5 dyed samples treated with the CAO/PEG1000 hybrid emulsion.
The fastness properties
The fastness properties of the dyed and dyed/finished fabric samples are represented by Table 9. Table 9 signifies that: (1) treating the dyed samples with nominated hybrid emulsion has no effect on washing fastness, dry rubbing fastness as well as acidic perspiration fastness of such samples, (2) the wet rubbing fastness and alkaline perspiration fastness of all the dyed/finished samples were enhanced, and (3) the light fastness of all the dyed/finished samples was decreased. The alteration in fastness properties of the dyed/finished fabric samples reflects the effect of deposition of the synthesized CAO/PEG hybrid ingredients onto the dyed samples structure.
CAO/PEG hybrids emulsion storage stability
Upon storing any of the aforementioned prepared hybrids emulsions for 1 month, tinny oil drops appear after the third day that by shaking a homogeneous emulsion is formed again. After one month, the emulsions separated into two layers; a turbid solution in the bottom and a thicker emulsion above it. The bottom layer height is about one third of the upper layer. However, upon shaking any of such emulsions, a homogeneous emulsion is reformed again.