Castor oil is a viscous, pale amber, non-edible oil that contains ricinoleic acid (C18H34O3, cis-12-hydroxy-9-octadecenoic acid) as its main component comprising 90% of its fatty acid component (Ogunniyi, 2006; Zhao et al., 2017). The hydroxyl group, double bond, and carboxyl group present in ricinoleic acid collectively function as pivotal reaction sites, enabling the synthesis of a diverse array of industrially valuable derivatives. These functional groups serve as catalysts for the synthesis of a broad spectrum of derivatives with significant industrial applications (Goswami et al., 2010). Ricinoleic acid can be converted into a variety of derivatives through an array of chemical modifications (Lee et al., 2016). The hydroxyl group present at the twelfth position in the ricinoleic acid molecule is responsible for the oil’s distinctive properties, such as being a desirable candidate for the synthesis of polyols and having greater oxidative stability. The oil has a relatively longer shelf life due to its higher viscosity (Singh et al., 2023). There are reports on numerous applications of ricinoleic acid such as surfactants, pigment for printing ink, plasticizers, lubricants, dye dispersers, textile finishing, heptaldehyde, azelaic acid, sebacic acid, and other valuable products. However, its main application is as a chemical intermediate in the production of various oleochemicals. Its various derivatives, such as ricinoleic alcohol, methyl ricinoleate, azelaic acid, suberic acid, and ricinenic acid have several industrial applications, especially for the preparation of emulsifiers, plasticizers, and soaps (Puthli et al., 2006). Some of its derivatives are used as antistatic and softening agents in hair and skin conditioners. Because of the intermolecular esterification of ricinoleic acid, which results in the formation of estolides, the standard high-temperature and high-pressure fatty acid manufacturing process is not suitable for castor oil hydrolysis. The ricinoleic acid market is expected to experience a Compound Annual Growth Rate (CAGR) of 4.59% from 2023 to 2028, with a projected increase in market size by USD 103.27 million. The expansion of this market is influenced by various factors, including increased demand from key end-users, simplified extraction and production methods for ricinoleic acid, and a rising focus on sustainable and environmentally friendly product options. Ricinoleic acid is typically synthesized through saponification followed by acidification (Puthli et al., 2006). Although the reaction conditions are mild in comparison to high-pressure hydrolysis for other oils, the ricinoleic acid produced from castor oil by this method has a distinct odor and color. Furthermore, using gentle operating conditions (Rajan et al., 2024) lipase catalyzed hydrolysis of castor oil can be performed. The enzymatic approach of hydrolysis can effectively overcome the drawbacks of conventional methods such as the significant production of the byproduct, Na2SO4 (Puthli et al., 2006). Lipase-catalyzed oil hydrolysis offers a key benefit in that it can generate superior-quality products in industrial processes that undergo immobilization on various carriers (Reyes-Reyes et al., 2022, Zhao et al., 2017). Consequently, a range of supports for lipase immobilization has been devised, encompassing synthetic resins, inorganic polymers, and synthetic organic polymers (Bié et al., 2022; Sheldon and van Pelt, 2013).
Magnetic nanoparticles (MNPs) have attracted significant interest as potential support carriers for enzyme immobilization in recent times, due to their expansive surface area and the existence of hydroxyl groups on their surface. These features enable easy functionalization and robust binding of enzyme molecules. The distinct traits of low porosity and outstanding mechanical stability play a vital role in minimizing steric hindrances, essential for establishing a stable enzyme-matrix catalytic system (Bilal et al., 2018; Li et al., 2013). Currently, it is understood that the main obstacles to the industrial application of lipase-catalyzed processes are the high costs of commercial immobilized lipases and their frequent insufficient operational stability. Therefore, finding new immobilization supports that encourage high activity and operational stability is necessary to create industrial enzymatic processes that are sustainable (Mota et al., 2020). The MNPs can be easily recovered and reused with an external magnetic force from the reaction media which is more effective than the centrifugation and filtration process (Rashid et al., 2021; Zhao et al., 2017). Biocompatibility, chemical stability, non-toxicity, and superparamagnetic qualities are further advantages of magnetic nanoparticle carriers that can increase the efficiency of enzyme-assisted operations at a lower cost (Verma et al., 2019; Mohamed et al., 2018). However, since they lack the proper functional groups, bare magnetic particles are unable to stabilize enzymes. For binding biomolecules, an extra inorganic or polymeric coating layer is required to get over this limitation (Zhao et al., 2017). Although simple to use, adsorption techniques are sensitive to pH variations. A significant issue with this approach is protein leaking. Using appropriate cross-linkers, covalent techniques can be used to solve this problem (Verma et al., 2019). Glutaraldehyde is widely recognized as one of the most extensively utilized protein crosslinking agents across diverse fields and is widely used for enzyme immobilization (Migneault et al., 2004). The glutaraldehyde groups added to the support surfaces can bind to the free amino groups on the supports and proteins. They can also interact with amino groups already linked to one glutaraldehyde molecule (Okura et al., 2020). Since the most reactive side chains of amino acids are nucleophiles, glutaraldehyde can react with a variety of protein functional groups, including amine, thiol, phenol, and imidazole (Migneault et al., 2004).
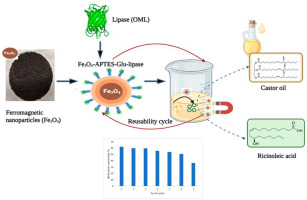
The ricinoleic acid formation by the hydrolysis of castor oil using the purified Pseudomonas guariconensis lipase with a molecular weight of 70 kDa (OML) has been reported in our previous study (Rajan et al., 2024). The lipase showed the highest affinity towards the long-chain fatty acids (especially with the fatty acid ricinoleic acid) and has stability towards metal ions, organic solvents, and alkaline pH conditions. These specificities of lipase can be explored for several biotechnological applications. The optimization of the production process is required for improved ricinoleic acid yield. The immobilized biocatalyst can significantly reduce the production cost by the repeated reusability during the production of ricinoleic acid. To our knowledge, this is the first study on the immobilization of Pseudomonas guariconensis lipase (OML) on glutaraldehyde functionalized ferromagnetic nanoparticle (Fe3O4-APTES-Glu-lipase) as a novel biocatalyst for the preparation of ricinoleic acid from castor oil. The schematic illustration of the process of immobilization the enzymatic conversion of castor oil and the reusability of the immobilized biocatalyst is shown in Fig. 1.