Introduction
Colorectal cancer (CRC) remains the major contributor to cancer-related morbidity and mortality (1). Colonoscopy been considered to be the preferred tool for effectively screening and early treating CRC (2). Issued data showed an approximate 50% reduction in mortality of CRC after resection of abnormal colonic lesions were performed by colonoscopy (3, 4). However, poor quality of bowel preparation will significantly decrease the efficacy and safety of colonoscopy procedure (5). Published data suggested that inadequate bowel preparation was directly associated with more than 40% of colonoscopy failures (6). Moreover, inadequate bowel preparation was related to lower polyp or adenoma detection rate (7), longer operation time (8), and higher risk of procedure related complications and incomplete colonoscopy rate (9).
To date, polyethylene glycol (PEG) solutions remain the preferred option of bowel preparation before colonoscopy (10). However, required high volume of liquid obviously reduces patients’ tolerability and compliance (11). Thus, adjunctive drugs such as bisacodyl and ascorbic acid (Asc) have been added into PEG solutions in order to minimize the required volume of liquid (10, 12). However, desired quality of bowel preparation has not already been achieved. Consequently, it remains an open question how to improve bowel preparation efficacy before colonoscopy.
Castor oil (CaO) was derived from the seed of Ricinus communishas and has been widely used as a safe and effective stimulant laxative for colon cleansing in many settings (13–16). For example, Apisarnthanarak et al. (13) detected comparative patients’ satisfaction and efficacy of colon cleansing between CaO and sodium phosphate. Yang et al. (16) unfolded that the laxative efficacy of CaO was comparable with that of bisacodyl. It is noted that the regime of bisacodyl plus PEG (17) and the regime of sodium and phosphate (18) achieved desired quality of bowel preparation, decreased the required volume of liquid, and improved compliance with the recommended regime when compared to standard PEG regime. Moreover, study also suggested that 2 L PEG containing Asc obtained similar bowel preparation efficacy with 3 L PEG (19), and which was superior to that of 2 L PEG with sodium phosphate (NaP) (20). So, we rationally speculated that 2 L PEG containing CaO may have comparative efficacy with 2 L PEG with bisacodyl or NaP in colon cleansing, and which is not inferior to high-volume 3 L PEG regime. Moreover, two trials (21, 22) suggested that 1 L PEG with bisacodyl and Asc was associated with improved patient’s tolerability and desired quality of bowel preparation compared with 2 L PEG with Asc. Consequently, we also speculated that CaO plus Asc may halve the required liquid of PEG solutions.
Previous studies (23–25) suggested a higher rate of adverse effects such as abdominal cramping, abdominal fullness, nausea, vomiting, fainting, and insomnia after orally taken a large dose 50 or 60 mL of CaO. However, some trials found that low dose 30 mL CaO did not obviously increase the incidence of adverse events (13, 26, 27). Thus, we hypothesized that 30 mL CaO may enhance colon cleansing of PEG, and 30 mL CaO plus Asc (28) may halve the required liquid volume of PEG. The aim of the present trial was to ascertain the efficacy and safety of 3 L PEG lavage solution, 2 L PEG lavage combined with CaO, and 1 L PEG lavage solution combined with CaO and Asc for preparation for colonoscopy.
Materials and Methods
Study Design
A single-center, randomized, observer-blinded three-arm study was conducted from October 2017 to December 2018 at the endoscopy center at Chongqing University Cancer Hospital (Chongqing, China). In total, 82 patients received lower volume 1 L PEG with CaO plus Asc (1 L-PEG-CaO-Asc), 84 received low volume 2 L PEG with CaO (2 L-PEG-CaO), or 80 patients received traditional volume 3 L PEG. At the time of registration, subjects were randomly assigned to one of three groups. They were randomized by a computer-generated list and were provided with written instructions. All patients provided written informed consent before taking part in the present study. We obtained ethical approval from the Ethics Committee of Chongqing University Cancer Hospital and Chongqing Cancer Hospital. The trial is registered at Chinese Clinical Trial Registry (www.chictr.org.cn) with identifier ChiCTR-IIR-17012418.
Patients
Inclusion Criteria
Participants met the following criteria were considered: (1) age above 18 and under 75 years; (2) adult outpatients who will be scheduled to morning colonoscopy regardless of sex; (3) did not participate in other clinical trials which also aimed at investigating bowel preparation efficacy; (4) agree to participate, and give signed written informed consents.
Exclusion Criteria
We excluded patients who met following criteria: (1) lactation; (2) pregnancy; (3) experienced the abdominal surgery such as gynecologic surgery, appendectomy, and laparoscopy; (4) neurological diseases; (5) contraindication of colonoscopy, (6) allergy to ingredients of PEG, CaO, or Asc or (6) other reasons such as uncontrolled severe hypertension and electrolyte imbalance that are considered to be unsuitable for study participation by the responsible investigators.
Recruitment, Randomization, and Blind
The direct investigators who have been certified for colonoscopy examination by Medical Committee of Chongqing University Cancer Hospital and have completed 3,000 examinations in colonoscopy assessed the eligibility of each candidate according to the inclusion and exclusion criteria the day before colonoscopy. After identified the eligibility, direct investigators instructed patients, their next of kin or legal representatives to complete the written informed consents.
Research team generated the random sequence using SPSS 22.0 software, and the random sequence was sealed in opaque envelope. Then, an independent research nurse to randomly divided recruited patients into one of the three groups as following on the basis of a table of random numbers: 3 L PEG group, 2 L-PEG-CaO group, and 1 L-PEG-CaO-Asc group. The day of colonoscopy, the research nurse case report form to collect demographic and clinical characteristics data of all eligible patients, which includes sex, age, body weight, body mass index, indications for colonoscopy, previous colonoscopy history, and comorbidity such as hypertension, diabetes mellitus, and cardiovascular disease.
In order to eliminate the risk of bias as much as possible, the endoscopists were blinded except the research nurse who conducted the randomization procedure during examine period. Moreover, research team also blinded the biostatistician.
Colonoscopy Preparation
According to the findings from our previous meta-analysis (29), all participants enrolled in our study were instructed to take low fat and residue diet without food color the 3 day before colonoscopy examination, and all started to fast at 20:00 pm on the day before colonoscopy examination. Patients were allowed eating bun, bread, and chocolate in order to enhance tolerance, decrease incidence of AEs such as hypoglycemia if they experienced serious hunger feeling. Moreover, investigators explained the purpose of colonoscopy and the importance of adequate bowel preparation before colonoscopy examination. In order to obtain adequate bowel preparation and take the fear away, investigators also explained the processes of bowel preparation for patients and the methods of processing all possible AEs associated with bowel preparation.
Moreover, the study protocols of all three groups have been outlined in our published protocol (30). According to the recommendation from US MultiSociety Task Force on Colorectal Cancer, we instructed all patients to ingest bowel preparation regime with split-dose. So, patients in 3 L PEG alone group were instructed to ingest 1 L PEG solution at 20:00 to 21:00 p.m. the day before colonoscopy and the remaining 2 L solution at the 3:00 to 5:00 a.m. on the morning before colonoscopy; patients in 2 L-PEG-CaO group were instructed to ingest 1 L PEG solution and 30 mL CaO at 20:00 to 21:00 p.m. the day prior to colonoscopy and the remaining 1 L PEG and extra 1 L clean water at the 3:00 to 5:00 a.m. on the morning before colonoscopy; patients in 1 L-PEG-CaO-Asc group were instructed to ingest 0.5 L PEG solution, 30 mL CaO, and 5 g Asc and extra 0.5 L clean water at 20:00 to 21:00 p.m. the day before colonoscopy and the remaining 0.5 L PEG solution and extra 1.5 L clean water at the 3:00 to 5:00 a.m. on the morning before colonoscopy. The patients were instructed to digest PEG solution 250 mL every 15 min. Moreover, all eligible participants were admitted to take oral 20 mL simethicone and 20 mL clean water at the 30 min prior to colonoscopy. For all eligible patients, propofol injection was intravenously administered for sedation.
Study Endpoints
Primary Outcome
We defined the bowel preparation efficacy and adequate bowel preparation rate as the primary outcome in the present study (30). The direct operation doctor used the Boston Bowel Preparation Scale (BBPS) to evaluate the quality of bowel preparation (31). BBPS is a comprehensive scoring system of evaluating bowel preparation efficacy before colonoscopy (31), and has been widely used in clinical practice worldwide (32, 33). Details of BBPS have been described in our published study protocol (30). In the present study, the quality of bowel preparation was defined as adequate when bowel preparation achieved excellent or good for each segment.
Secondary Outcomes
We defined cecal intubation time, withdraw time, cecal intubation success rate, detection rate of polyp and adenoma, patients’ satisfaction, tolerability and willingness to repeat colonoscopy, and quality of sleep as secondary outcomes. Cecal intubation time (endoscopists recorded the time of started colonoscopy examination until colonoscopy reached ileocecal part), withdraw time (endoscopists recorded the time of completely withdrew colonoscopy from anus), cecal intubation success rate (the proportion of successfully reached ileocecal part), and detection rate of polyp and adenoma (the proportion of polyp and adenoma detecte in the whole colonoscopy procedure) were measured by direct investigator. Patients’ satisfaction (patients answered the questioner through selecting yes or no), tolerability [patients expressed feeling with a Likert scale ranged from 1 (not good) to 4 (excellent)] and willingness to repeat colonoscopy (patients expressed feeling to repeat colonoscopy through selecting yes or no), and quality of sleep (patients were instructed to self-evaluate the quality of sleep through answering the same or worse when it was compared to the previous night’s sleep quality) were evaluated by research nurse with self-designed questionner.
Safety Assessments
The direct investigator recorded all AEs related to bowel preparation and colonoscopy such as abdominal fullness, abdominal pain, nausea, vomiting, and others into the case report form. It is noted that any symptom that existed before the start of the bowel preparation was not be recorded as AEs.
Sample Size and Statistical Analysis
The bowel preparation efficacy was primarily tested in the present study, and thus we calculated the anticipated sample size based on this outcome. Based on the findings from previous studies (19, 21), we proposed that the rate of adequate bowel preparation in 3 L PEG, 2 L PEG with 30 mL CaO, and 1 L PEG with 30 mL CaO plus Asc will be 85, 90, and 95%. We assumed the significance and power to be 0.05 and 80%, respectively, and thus the sample size required to detect a difference will be 255 patients according to the non-inferiority design. Because the dropout rate was expected to be 10%, each trial group will be made up of at least 94 participants.
Continuous variables were expressed as mean ± standard deviation (SD), and discontinuous variables were expressed as counts and percentages. Data were analyzed on a Full Analysis Set basis with SPSS for Windows release 22.0 software (SPSS, Chicago, Illinois, USA). χ2 analysis or Fisher’s exact test was used for comparison of categorical data. Normally distributed continuous data were analyzed by means of one-way ANOVA. Kruskal–Wallis H-test was used only for analysis of non-normally distributed data. Differences were considered significant at P < 0.050.
Results
Baseline Characteristics
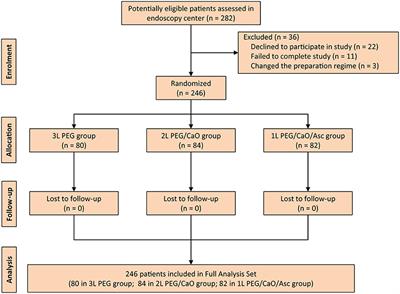
During the study period, a total of 282 consecutive patients were screened, but 36 patients were excluded due to various reasons including declined to participate in study (22 patients), failed to complete study (11 patients), and changed the preparation regime (three patients). Therefore, 246 patients were randomized and included in the full analysis set (FAS). A flow diagram that describes patients’ enrollment is depicted in Figure 1.
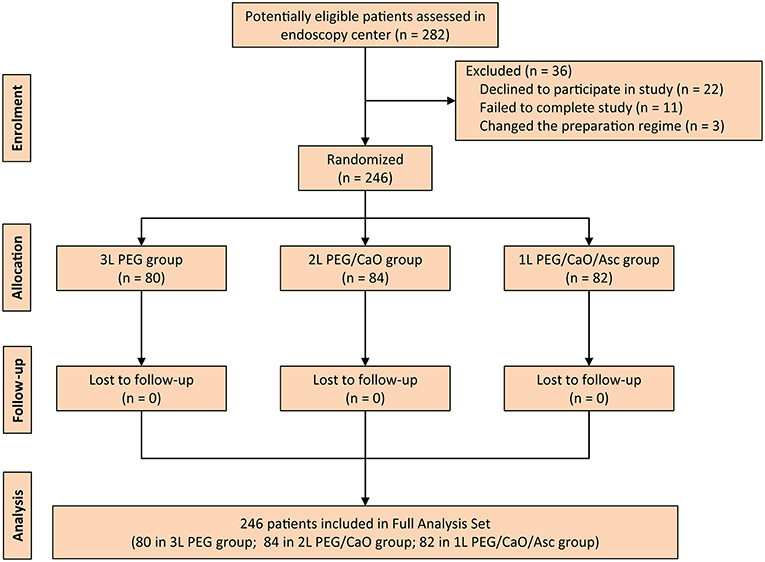
Figure 1. CONSORT diagram for the trial. PEG, polyethylene glycol; CaO, castor oil; Asc, ascorbic acid.
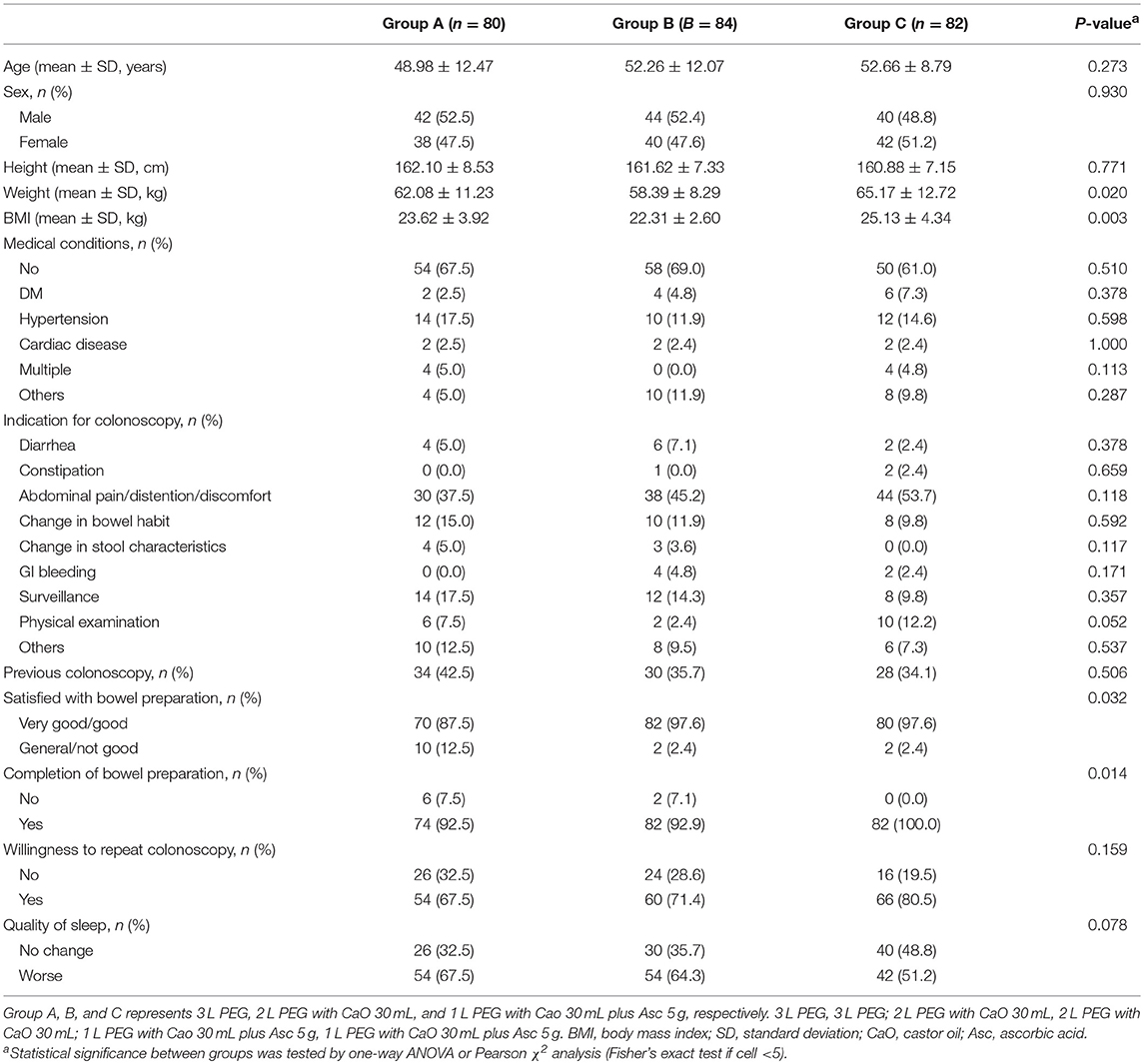
The three groups were comparable in terms of age, sex, height, medical conditions, the indication for colonoscopy, previous colonoscopy, willingness to repeat colonoscopy and quality of sleep (Table 1). The weight (p = 0.020) and body mass index (BMI) (P = 0.003) in 3 L PEG and 1 L PEG-CaO-Asc groups were higher than that in 2 L PEG-CaO group. The most common reasons for colonoscopy were abdominal pain/distention/discomfort, changed in bowel habit, and post-polypectomy surveillance.
Primary Outcome
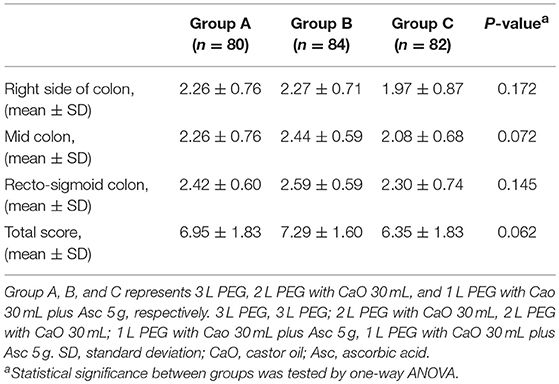
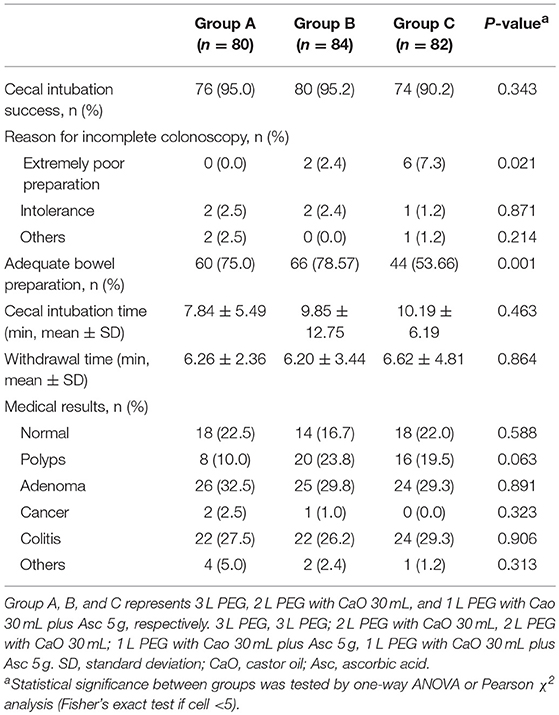
All methods showed no significant difference in terms of quality of bowel preparation scoring, with a mean (SD) total score of 6.95 ± 1.83 for the 3 L PEG group, 7.29 ± 1.60 for 2 L PEG-CaO-Asc group and 6.35 ± 1.83 for the 1 L PEG-CaO-Asc (P = 0.062). The analysis of the segmental (right, mid, and recto-sigmoid colon) BBPS scale showed no difference for the right side (2.26 ± 0.76 vs. 2.27 ± 0.71 vs. 1.97 ± 0.8), mid colon (2.26 ± 0.76 vs. 2.44 ± 0.59 vs. 2.08 ± 0.6), and recto-sigmoid colon (2.42 ± 0.60 vs. 2.59 ± 0.59 vs. 2.30 ± 0.74). Table 2 presents the results of bowel cleansing quality assessment based on the BBPS. Percent of adequate bowel preparation, defined as total BBPS score ≥6 was 75.0, 78.57, and 53.6% in 3 L PEG, 2 L PEG-CaO, and 1 L PEG-CaO-Asc groups, and the difference was statistically significant (p = 0.001) (Figure 2). Further analysis based on paired comparison found that 3 L PEG (P = 0.010) and 2 L PEG-CaO (P = 0.002) significantly increased the percent of adequate bowel preparation compared to 1 L PEG-CaO-Asc regime (Figure 2 and Table 3). Moreover, the reasons for incomplete colonoscopy mainly were extremely poor preparation and intolerance, however the percent of extremely poor preparation in 1 L PEG-CaO-Asc group was significantly higher than that in 3 L PEG (7.3 vs. 0.0%) and 2 L PEG-CaO (7.3 vs. 2.4%) groups.
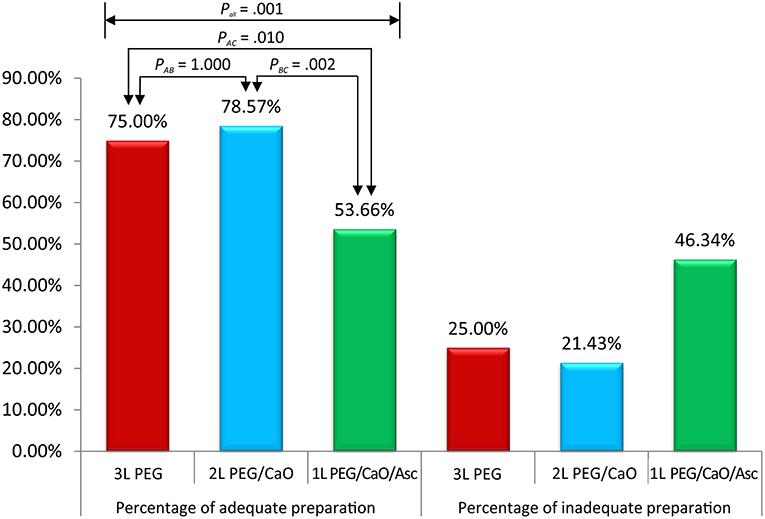
Figure 2. Percentages of adequate and inadequate bowel preparation among three groups. Pall corresponded to comparison of three groups, PAB corresponded to comparison between 3 L PEG and 2 L PEG/CaO groups, PAC corresponded to comparison between 3 L PEG and 1 L PEG/CaO/Asc groups, and PBC corresponded to comparison between 2 L PEG/CaO and 1 L PEG/CaO/Asc groups. All comparisons were tested by one-way ANOVA or Pearson χ2 analysis (Fisher’s exact test if cell <5). A, B, and C represents 3 L PEG, 2 L PEG/CaO, and 1 L PEG/CaO/Asc, respectively. PEG, polyethylene glycol; CaO, castor oil; Asc, ascorbic acid.
Secondary Outcome
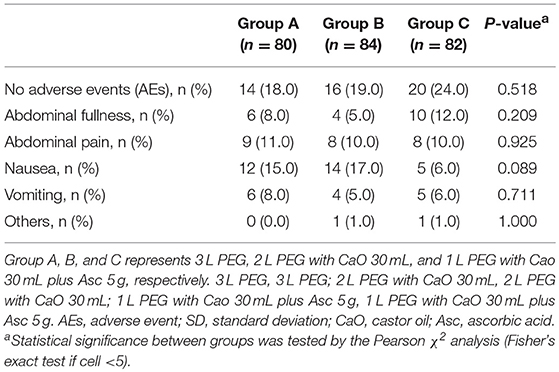
Details of colonoscopy procedures are summarized in Table 3, the cecal intubation rate of all groups was >90%, the insertion time was about 6 min, and the average withdrawal time was >7 min. The endoscopic diagnoses of the three groups were comparable, about 20% of the patients had no abnormal findings, over 20% of the patients were found to have colitis, over 40% of the patients were found to have colorectal polyps, in addition, more than 55% of the polyps were adenomas. Only very few patients had cancer (3/246, 1.2%). Patients in 2 L PEG-CaO and 1 L PEG-CaO-Asc groups were more satisfied with the process of bowel preparation than patients in 3 L PEG group (97.6 vs. 97.6 vs. 87.5%, p = 0.032). 92.5% and 92.9% patients in 3 L PEG and 2 L PEG-CaO groups completed bowel preparation, whereas all the patients in 1 L PEG-CaO-Asc group accomplished bowel preparation (p = 0.014). In addition, only 67.5% patients in 3 L PEG group were willing to repeat colonoscopy in the endoscopy center if necessary, but 71.4 and 80.5% patients in 2 L PEG-CaO and 1 L PEG-CaO-Asc groups were willing to do so although significant results were not detected (P = 0.159). There were no significant differences in quality of sleep (Table 1), the rates of abdominal fullness, abdominal pain, nausea, vomiting, and other AEs among three groups (Table 4).
Discussion
Although many novel and promising approaches have been proposed, colonoscopy remains a routine method of screening and early treating CRC (2). However, the quality of bowel preparation will significantly affect the efficacy and safety of colonoscopy examination (5), and evidence suggests about 25% of inadequate bowel preparation before colonoscopy (5). It must be important to note that poor bowel preparation will also increase the rate of incomplete colonoscopy and adverse events and lower polyp and adenoma detection rate (34, 35). Thus, several methods have been proposed to improve the quality of bowel preparation (36–38). Of these all methods, PEG solutions remain the first-line recommendation for bowel preparation prior to colonoscopy due to desire laxative efficacy (10), however required high volume of liquid will reduce tolerability and compliance to bowel preparation (11). Thus, numerous studies have been performed to explore the potential of reducing the volume of the cleansing solution by adding adjunctive prokinetics such as bisacodyl (39, 40), but the evidence suggests that gastrointestinal prokinetics can induce dose-dependent cardiac adverse effects (41). So, it is important to find a novel adjunctive laxative.
CaO is extracted from the seed of the castor-oil plant (42). CaO has a high content of the hydroxylated unsaturated fatty acid ricinoleic acid (43), and it has been demonstrated that released ricinoleic acid has the ability of inducing strong laxative effect by activating small-intestinal smooth-muscle cells via the EP3 prostanoid receptor (44). Moreover, CaO will not cause serious side effects (27), and thus it has been used as a safe stimulant laxative in many settings (14–16) except for pregnant women (45). Evidence suggested low dose CaO (30 mL) has similar laxative efficacy of cleaning colon to bisacodyl (16). Moreover, studies (26, 27) also showed that 30 mL of CaO can reduce volume of preparation of bowel preparation solutions. And thus, we designed a regime of 2 L split PEG with 30 mL CaO to perform bowel preparation before colonoscopy. The findings of our randomized controlled trial suggested that 2 L split PEG plus 30 mL CaO can increase the adequate bowel preparation rate and patients’ satisfaction toward and patients’ compliance with regime with comparable BBPS score and AEs rate compared to traditional 3 L split PEG solution.
A number of studies found that PEG with Asc regime obtained comparative efficacy, acceptability, tolerability, and safety related to the standard PEG regime (6, 11, 46, 47). Moreover, a recent meta-analysis also demonstrated the efficacy and safety of low-volume PEG containing Asc regime for bowel cleansing (48). Asc produces cathartic effects because of it will become saturated at a high dose (49, 50). Asc contribute toward decreasing the total volume of PEG solution required for gut lavage and improve patient’s tolerability (27). For these reasons, we have further designed a lower-volume PEG preparation with 30 mL CaO plus 5 g Asc. The finding of our study showed that this modified lower-volume PEG regime obtained higher patients’ satisfaction and compliance. However, it is noted that this modified bowel preparation regime significantly decreased the adequate bowel preparation rate compared with traditional 3 L split PEG regime. In the present study, patients with higher BMI were assigned to oral ingestion of 1 L split PEG with 30 mL CaO plus 5 g Asc. Studies have found that high BMI is an independent factor associated with inadequate bowel preparation for colonoscopy (51–53). This difference may be the contributor to the inconsistent finding. So, further studies are needed in order to determine the adjunctive efficacy of combination of CaO and Asc.
We must acknowledge that our study has some limitations. First, in this study, evaluation on electrolyte levels and hematological analysis were not performed either before or after colon preparation. However, no patient experienced significant adverse events related to bowel preparation and procedure. Second, we performed this study in single-center and obtained results supported by insufficient number of patients. Thus, we suggest to design a multi-center study with larger scale to perform a more precise assessment. Third, evaluation of electrolyte levels or hematological analysis was not carried out during the whole colonoscopy examination. However, we did not detect any significant difference in the rate of adverse events.
Conclusions
In conclusion, the results of this study indicate that 30 mL CaO in addition to 2 L PEG before colonoscopy is safe, and it can improve patients’ satisfaction toward and compliance with the process of bowel preparation, increase the adequate bowel preparation rate, and obtain equal quality of bowel preparation to 3 L split PEG. Both preparation methods were effective. Patient’s adverse events and quality of sleep were similar between the two groups. However, patients taking 1 L PEG with 30 mL CaO plus Asc 5 mg in general showed more inadequate bowel preparation although it improved patients’ satisfaction and compliance related to 3 L PEG regime.
Data Availability
The raw data supporting the conclusions of this manuscript will be made available by the authors, without undue reservation, to any qualified researcher.
Ethics Statement
All patients provided written informed consent before taking part in the present study. We obtained ethical approval from the Ethics Committee of Chongqing University Cancer Hospital and Chongqing Cancer Hospital.
Author Contributions
XT and W-QC: conceptualization and resources. XT and X-LL: data curation. XT: formal analysis, software, and visualization. W-QC: funding acquisition, project administration, supervision, writing—review, and editing. XT, BS, and HC: investigation. XT and BS: methodology. XT, BS, X-LL, and W-QC: validation. XT and HC: writing—original draft.
Funding
This research was funded by the Clinical Research Foundation of Chongqing University Cancer Hospital and Chongqing Cancer Hospital (grant number: LY2017004).
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We expressed the warm appreciation to all patients voluntarily participated in our clinical trial.
References
2. Tian X, Chen WQ, Huang JL, He LY, Liu BL, Liu X, et al. Effects of polyethylene glycol 2 L alone or with ascorbic acid compared with polyethylene glycol 4 L alone for bowel preparation before colonoscopy: protocol for a systematic review and network meta-analysis. BMJ Open. (2017) 7:e018217. doi: 10.1136/bmjopen-2017-018217
3. Quintero E, Castells A, Bujanda L, Cubiella J, Salas D, Lanas A, et al. Colonoscopy versus fecal immunochemical testing in colorectal-cancer screening. N Engl J Med. (2012) 366:697–706. doi: 10.1056/NEJMoa1108895
4. Zauber AG, Winawer SJ, O’Brien MJ, Lansdorp-Vogelaar I, van Ballegooijen M, Hankey BF, et al. Colonoscopic polypectomy and long-term prevention of colorectal-cancer deaths. N Engl J Med. (2012) 366:687–96. doi: 10.1056/NEJMoa1100370
5. Froehlich F, Wietlisbach V, Gonvers JJ, Burnand B, Vader JP. Impact of colonic cleansing on quality and diagnostic yield of colonoscopy: the European panel of appropriateness of gastrointestinal endoscopy European multicenter study. Gastrointest Endosc. (2005) 61:378–84. doi: 10.1016/S0016-5107(04)02776-2
6. Ponchon T, Boustiere C, Heresbach D, Hagege H, Tarrerias AL, Halphen M. A low-volume polyethylene glycol plus ascorbate solution for bowel cleansing prior to colonoscopy: the NORMO randomised clinical trial. Dig Liver Dis. (2013) 45:820–6. doi: 10.1016/j.dld.2013.04.009
7. Lebwohl B, Kastrinos F, Glick M, Rosenbaum AJ, Wang T, Neugut AI. The impact of suboptimal bowel preparation on adenoma miss rates and the factors associated with early repeat colonoscopy. Gastrointest Endosc. (2011) 73:1207–14. doi: 10.1016/j.gie.2011.01.051
8. Harewood GC, Sharma VK, de Garmo P. Impact of colonoscopy preparation quality on detection of suspected colonic neoplasia. Gastrointest Endosc. (2003) 58:76–9. doi: 10.1067/mge.2003.294
9. Rex DK, Imperiale TF, Latinovich DR, Bratcher LL. Impact of bowel preparation on efficiency and cost of colonoscopy. Am J Gastroenterol. (2002) 97:1696–700. doi: 10.1111/j.1572-0241.2002.05827.x
10. Clark RE, Godfrey JD, Choudhary A, Ashraf I, Matteson ML, Bechtold ML. Low-volume polyethylene glycol and bisacodyl for bowel preparation prior to colonoscopy: a meta-analysis. Ann Gastroenterol. (2013) 26:319–24.
11. Choi HS, Shim CS, Kim GW, Kim JS, Lee SY, Sung IK, et al. Orange juice intake reduces patient discomfort and is effective for bowel cleansing with polyethylene glycol during bowel preparation. Dis Colon Rectum. (2014) 57:1220–7. doi: 10.1097/DCR.0000000000000195
12. Valiante F, Pontone S, Hassan C, Bellumat A, De Bona M, Zullo A, et al. A randomized controlled trial evaluating a new 2-L PEG solution plus ascorbic acid vs. 4-L PEG for bowel cleansing prior to colonoscopy. Dig Liver Dis. (2012) 44:224–7. doi: 10.1016/j.dld.2011.10.007
13. Apisarnthanarak P, Rotjanaaree B, Komoltri C, Charoensak A, Apisarnthanarak A, Hargrove NS. Prospective, randomized comparison of castor oil and sodium phosphate preparation for barium enema. J Med Assoc Thai. (2009) 92:243–9.
15. Jansson M, Geijer H, Andersson T. Bowel preparation for excretory urography is not necessary: a randomized trial. Br J Radiol. (2007) 80:617–24. doi: 10.1259/bjr/78311002
16. Yang HC, Sheu MH, Wang JH, Chang CY. Bowel preparation of outpatients for intravenous urography: efficacy of castor oil versus bisacodyl. Kaohsiung J Med Sci. (2005) 21:153–8. doi: 10.1016/S1607-551X(09)70294-3
17. Brahmania M, Ou G, Bressler B, Ko HK, Lam E, Telford J, et al. 2 L versus 4 L of PEG3350 + electrolytes for outpatient colonic preparation: a randomized, controlled trial. Gastrointest Endosc. (2014) 79:408–16.e4. doi: 10.1016/j.gie.2013.08.035
18. Bae SE, Kim KJ, Eum JB, Yang DH, Ye BD, Byeon JS, et al. A Comparison of 2 L of polyethylene glycol and 45 mL of sodium phosphate versus 4 L of polyethylene glycol for bowel cleansing: a prospective randomized trial. Gut Liver. (2013) 7:423–9. doi: 10.5009/gnl.2013.7.4.423
19. Zhang S, Li M, Zhao Y, Lv T, Shu Q, Zhi F, et al. 3-L split-dose is superior to 2-L polyethylene glycol in bowel cleansing in Chinese population: a multicenter randomized, controlled trial. Medicine. (2015) 94:e472. doi: 10.1097/MD.0000000000000472
20. Cohen SM, Wexner SD, Binderow SR, Nogueras JJ, Daniel N, Ehrenpreis ED, et al. Prospective, randomized, endoscopic-blinded trial comparing precolonoscopy bowel cleansing methods. Dis Colon Rectum. (1994) 37:689–96. doi: 10.1007/BF02054413
21. Ji EK, Lee JW, Im JP, Ji WK, Su HK, Koh SJ, et al. Comparable efficacy of a 1-L PEG and ascorbic acid solution administered with bisacodyl versus a 2-L PEG and ascorbic acid solution for colonoscopy preparation: a prospective, randomized and investigator-blinded trial. PLoS ONE. (2016) 11: e0162051. doi: 10.1371/journal.pone.0162051
22. Kang SH, Lee JH, Yoo IK, Lee JM, Kim SH, Choi HS, et al. Sa1472 A comparison of bowel preparation between 2L ascorbic acid mixed PEG and 1L ascorbic acid mixed PEG with bisacodyl. Gastrointest Endosc. (2016) 83:AB287. doi: 10.1016/j.gie.2016.03.448
23. Avgerinos A, Kalantzis N, Rekoumis G, Pallikaris G, Arapakis G, Kanaghinis T. Bowel preparation and the risk of explosion during colonoscopic polypectomy. Gut. (1984) 25:361. doi: 10.1136/gut.25.4.361
24. Chen CC, Ng WW, Chang FY, Lee SD. Magnesium citrate-bisacodyl regimen proves better than castor oil for colonoscopic preparation. J Gastroenterol Hepatol. (1999) 14:1219–22. doi: 10.1046/j.1440-1746.1999.02032.x
25. Ghazikhanlou SK, Jafari MR, Shams S. A comparison of the efficacy, adverse effects, and patient compliance of the sena-graph®syrup and castor oil regimens for bowel preparation. Iran J Pharm Res. (2010) 9:193–8.
26. Hotta N. The use of castor oil for bowel preparation for colon capsule endoscopy. Open J Med Imaging. (2016) 06:103–7. doi: 10.4236/ojmi.2016.64010
27. Ohmiya N, Hotta N, Mitsufuji S, Nakamura M, Omori T, Maeda K, et al. Multicenter feasibility study of bowel preparation with castor oil for colon capsule endoscopy. Dig Endosc. (2018) 31:164–72. doi: 10.1111/den.13259
28. Rivas JM, Perez A, Hernandez M, Schneider A, Castro FJ. Efficacy of morning-only 4 liter sulfa free polyethylene glycol vs 2 liter polyethylene glycol with ascorbic acid for afternoon colonoscopy. World J Gastroenterol. (2014) 20:10620–7. doi: 10.3748/wjg.v20.i30.10620
29. Song GM, Tian X, Ma L, Yi LJ, Shuai T, Zeng Z, et al. Regime for bowel preparation in patients scheduled to colonoscopy: low-residue diet or clear liquid diet? evidence from systematic review with power analysis. Medicine. (2016) 95:e2432. doi: 10.1097/MD.0000000000002432
30. Tian X, Chen WQ, Liu XL, Chen H, Liu BL, Pi YP. Comparative efficacy of combination of 1 L polyethylene glycol, castor oil and ascorbic acid versus 2 L polyethylene glycol plus castor oil versus 3 L polyethylene glycol for colon cleansing before colonoscopy. Medicine. (2018) 97:e0481. doi: 10.1097/MD.0000000000010481
33. Gao Y, Lin JS, Zhang HD, Lin MX, Cheng CS, Wu SZ. Pilot validation of the Boston bowel preparation scale in China. Dig Endosc. (2013) 25:167–73. doi: 10.1111/j.1443-1661.2012.01356.x
34. Chokshi RV, Hovis CE, Hollander T, Early DS, Wang JS. Prevalence of missed adenomas in patients with inadequate bowel preparation on screening colonoscopy. Gastrointest Endosc. (2012) 75:1197–203. doi: 10.1016/j.gie.2012.01.005
35. Clark BT, Tarun R, Loren L. What level of bowel prep quality requires early repeat colonoscopy: systematic review and meta-analysis of the impact of preparation quality on adenoma detection rate. Am J Gastroenterol. (2014) 109:1714–23. doi: 10.1038/ajg.2014.232
36. Chang CW, Shih SC, Wang HY, Chu CH, Wang TE, Hung CY, et al. Meta-analysis: the effect of patient education on bowel preparation for colonoscopy. Endosc Int Open. (2015) 3:E646–52. doi: 10.1055/s-0034-1392365
37. Lichtenstein GR, Cohen LB, Uribarri J. Review article: Bowel preparation for colonoscopy–the importance of adequate hydration. Aliment Pharmacol Ther. (2010) 26:633–41. doi: 10.1111/j.1365-2036.2007.03406.x
38. Myriam M, Barkun AN, Charles M, Sophie R, Omar K, Alain V. Split-dose preparations are superior to day-before bowel cleansing regimens: a meta-analysis. Gastroenterology. (2015) 149:79–88. doi: 10.1053/j.gastro.2015.04.004
39. Martínek J, Hess J, Delarive J, Jornod P, Blum A, Pantoflickova D, et al. Cisapride does not improve precolonoscopy bowel preparation with either sodium phosphate or polyethylene glycol electrolyte lavage. Gastrointest Endosc. (2001) 54:180–5. doi: 10.1067/mge.2001.116562
40. Tajika M, Niwa Y, Bhatia V, Kawai H, Kondo S, Sawaki A, et al. Efficacy of mosapride citrate with polyethylene glycol solution for colonoscopy preparation. World J Gastroenterol. (2012) 18:2517–25. doi: 10.3748/wjg.v18.i20.2517
41. Tonini M, De Ponti F, Di Nucci A, Crema F. Review article: cardiac adverse effects of gastrointestinal prokinetics. Aliment Pharmacol Ther. (1999) 13:1585–91. doi: 10.1046/j.1365-2036.1999.00655.x
44. Tunaru S, Althoff TF, Nüsing RM, Martin D, Stefan O. Castor oil induces laxation and uterus contraction via ricinoleic acid activating prostaglandin EP3 receptors. Proc Natl Acad Sci USA. (2012) 109:9179–84. doi: 10.1073/pnas.1201627109
46. Marmo R, Rotondano GG, Marone A, Bianco MA, Stroppa I, Caruso A, et al. Effective bowel cleansing before colonoscopy: a randomized study of split-dosage versus non-split dosage regimens of high-volume versus low-volume polyethylene glycol solutions. Gastrointest Endos. (2010) 72:313–20. doi: 10.1016/j.gie.2010.02.048
47. Corporaal S, Kleibeuker JH, Koornstra JJ. Low-volume PEG plus ascorbic acid versus high-volume PEG as bowel preparation for colonoscopy. Scand J Gastroenterol. (2010) 45:1380–6. doi: 10.3109/00365521003734158
48. Xie Q, Chen L, Zhao F, Zhou X, Huang P, Zhang L, et al. A meta-analysis of randomized controlled trials of low-volume polyethylene glycol plus ascorbic acid versus standard-volume polyethylene glycol solution as bowel preparations for colonoscopy. PLoS ONE. (2014) 9:e99092. doi: 10.1371/journal.pone.0099092
49. Flavio Valiante Angelo Bellumat Manuela Bona, et al. Bisacodyl plus split 2-L P-citrate-simethicone improves quality of bowel preparation before screening colonoscopy. World J Gastroenterol. (2013) 19:5493–9. doi: 10.3748/wjg.v19.i33.5493
50. Tajika M, Tanaka T, Ishihara M, Mizuno N, Hara K, Hijioka S, et al. A randomized controlled trial evaluating a low-volume peg solution plus ascorbic acid versus standard PEG solution in bowel preparation for colonoscopy. Gastroenterol Res Pract. (2015) 2015:1–12. doi: 10.1155/2015/326581
51. Fayad NF, Kahi CJ, Abd El-Jawad KH, Shin AS, Shah S, Lane KA, et al. Association between body mass index and quality of split bowel preparation. Clin Gastroenterol Hepatol. (2013) 11:1478–85. doi: 10.1016/j.cgh.2013.05.037
52. Hyun JH, Kim SJ, Park JH, Wie GA, Kim JS, Han KS, et al. Lifestyle factors and bowel preparation for screening colonoscopy. Ann Coloproctol. (2018) 34:197–205. doi: 10.3393/ac.2018.03.13