With substantial progress made towards understanding disease management and treatment in relation to genetic variations, personalized medicine allows for the design of treatment protocols with greater precision (Chan and Ginsburg, 2011). One dosage form that holds a great potential for personalized medicine is the multi-unit pellet system (MUPS). MUPS comprises multi-particulates, usually as pellets or spheroids of 0.5–1 mm in diameter, which confers flexibility to dose customization in therapeutics. MUPS may contain multiple drug types loaded in different pellets which may sometimes be chemically incompatible, to be combined for single dose administration, thereby reducing the pill burden of patients (Abdul et al., 2010). In addition, the overall drug release profile can be modified by combining pellets with and without diffusion barrier coatings. It is also possible to customize the delivery of drugs to different sites in the gastrointestinal tract by the application of erodible or pH-sensitive coatings onto the pellets. There is also reduced risk of dose dumping, less dependence on gastric emptying rate, and minimized local irritation. Clearly, the versatility of MUPS poses it as an attractive dosage form, particularly for the future development of personalized medicine.
The rational design of MUPS with different release profiles demands a thorough understanding of the formulation and processing requirements needed to achieve and optimize a particular drug release profile. Site-specific intestinal release can be achieved by the application of coatings using enteric polymers which exhibit pH-dependent solubility such as hydroxypropyl methylcellulose acetate succinate. For a sustained release profile, diffusion barrier coatings with water-insoluble polymers such as cellulose acetate or ethylcellulose can be used. Another popular coating polymer is the methacrylic acid copolymer and the variation in the substituent groups can be useful to formulate sustained release products. The use of some methacrylic acid copolymers such as the ethyl acrylate and methyl methacrylate copolymer or the methacrylic acid and ethyl acrylate copolymer is particularly favored for coating pellets to be used in the production of MUPS tablets due to their plastically deforming nature (Partheniadis et al., 2020), which makes them less susceptible to compression-induced coat damage compared to the more brittle cellulosic polymers (Bodmeier and Paeratakul, 1994, Hiew et al., 2020). While the versatility of methacrylic acid copolymers makes them pliable for pellet coating, there are impediments associated with their use. When used as aqueous polymer dispersion, a plasticizer is often required to reduce the minimum film formation temperature of the polymer through the depression of the overall glass transition temperature, in order to facilitate coalescence of the polymer particles during the drying and curing process (Banker, 1966, Lecomte et al., 2004). Studies have shown that the type and concentration of plasticizer played a contributory role in controlling drug release (Schultz et al., 1997). For example, the use of water-soluble triethyl citrate (TEC), as opposed to the more lipophilic dibutyl phthalate, resulted in faster drug release (Maul and Schmidt, 1995) due to leaching of TEC (Bando and McGinity, 2006). Higher lipophilic plasticizer concentrations had also been associated with forming mechanically weaker films. The lipophilic plasticizer, dibutyl sebacate, had been reported to weaken film strength to a larger extent than TEC (Bussemer et al., 2003). While there is a clear need to plasticize the methacrylic acid copolymer for better film formation, adding a plasticizer is also associated with tackiness during coating and curing. Thus, a solution to overcome tackiness often necessitates the addition of an anti-adherent. Talc is a commonly used anti-adherent as it has been shown to be effective with acrylic polymers (Ammar et al., 2016, Felton and McGinity, 2002). However, talc can sometimes sediment during the coating process or cause choking of the spray nozzle. Additionally, talc has been reported to weaken the film strength, especially when elongated or stretched (Heinicke and Schwartz, 2007).
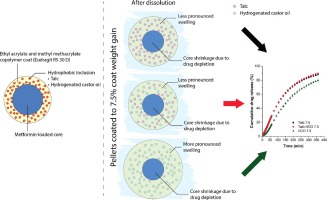
With coated particles such as pellets, the semi-permeable polymer membrane permits the influx of water to dissolve the water-soluble components within but limits the efflux of these components, thereby modifying their release rate. Some of the factors that affect the rate of efflux include the membrane thickness and tortuosity (Borgquist et al., 2002, Ozturk et al., 1990) and the mechanical strength of the membrane (Jensen et al., 1995). With highly water-soluble drugs, a saturated solution quickly forms following water influx and exerts substantial osmotic pressure, thereby creating an osmotic pressure gradient between the core of the pellets and the external dissolution media. This osmotic pressure difference drives drug release across the semi-permeable membrane (Ozturk et al., 1990, Qiu and Lee, 2017). A previous study reported that the addition of stearic acid and hydrogenated castor oil (HCO) as hydrophobic inclusions to ethylcellulose film coat slowed the rate of drug release (Hiew et al., 2019). As talc has been reported to help to retard the drug release of methacrylic acid copolymer coats, the current study was designed to investigate if HCO can serve as an alternative to talc, especially with highly water-soluble drugs.
The objective of this study was to investigate how an additive impacts the sustained release coat’s ability to withstand the osmotic pressure in the pellet core. HCO was chosen as the additive to be studied as it contains both hydrogen bond donor and receptor, which enables it to interact chemically with the coating polymer (Manatunga et al., 2022, Ogunniyi, 2006). In the present study, metformin hydrochloride was chosen as a model highly water-soluble drug. Pellets were prepared using extrusion-spheronization and coated with methacrylic acid copolymer containing 29 %, w/w of talc, HCO, or talc and HCO in a 1:1 ratio (w/w) using a fluid bed coater to different coat weight gains. Drug release from the coated pellets was monitored using the USP dissolution apparatus 2 and from single pellets using the surface dissolution imager. Pellet swelling was determined by measuring the change with time of the wetted pellet’s diameter through imaging.