Structural analysis of SMPUs
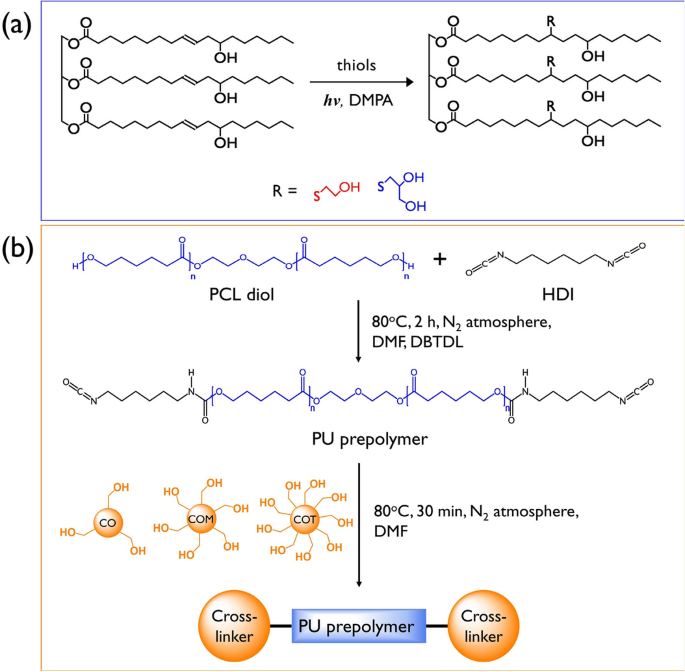
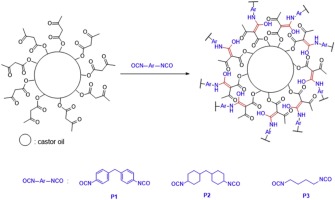
SMPUs were successfully synthesized by applying PCL-diol (2000 g/mol) as a soft segment and either CO or the as-prepared COM or COT as a crosslinker. As shown in Fig. 1b, the two-step synthesis was carried out by a prepolymer synthesis step and a crosslinking formation step. Three types of transparent and flexible PU samples were obtained.
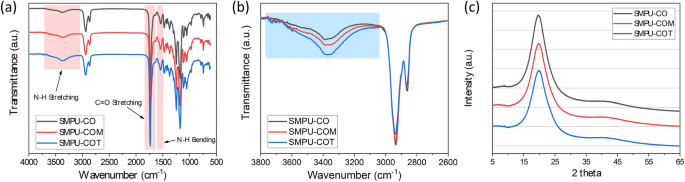
To analyze the chemical structures of the synthesized SMPUs, FT-IR analysis was performed; the results are shown in Fig. 2. Based on the full FT-IR spectra of the SMPUs (Fig. 2a), the success of the syntheses of SMPU-CO, SMPU-COM, and SMPU-COT was confirmed by the disappearance of the isocyanate peak of HDI, which is known to appear near 2265 cm−1. Compared to the PCL-diol spectrum, the spectra of the SMPU samples showed new peaks corresponding to N–H and C=O stretching bands in the urethane bonding at 3380 and 1728 cm−1, respectively, and a peak ascribed to N–H bending at 1533 cm−1. Moreover, peaks due to the symmetric sp2 and asymmetric sp3 stretching bands from the aliphatic chain were observed at 2937 and 2859 cm−1, respectively25. No significant change in the FT-IR spectra was observed after introducing the crosslinking agents with different OH values into the hard segment. According to several studies, however, we can obtain meaningful information about hydrogen bonding in PUs from the FT-IR spectra26. The most widely known approach is to determine the amount of hydrogen bonds by separating the carbonyl peaks. In the PCL-based PU, it is not straightforward to separate the hydrogen-bonded carbonyl peak (about 1700 cm−1) and the free carbonyl peak (about 1730 cm−1)27. In our study, the carbonyl peaks were also overlapped because of the carbonyl peaks of PCL. In such a case, however, the N–H stretching peak can provide meaningful information on hydrogen bonding. It has been found that the sharper the N–H stretching peak, the greater the number of hydrogen bonds; whereas, the broader it is, the fewer hydrogen bonds. The magnified N–H stretching peaks of the SMPU samples are shown in Fig. 2b. The N–H stretching peak was broadened in the order of CO < COM < COT. Based on this, the number of hydrogen bonds in the PU decreased in the order of SMPU-CO > SMPU-COM > SMPU-COT. This phenomenon can be explained by the fact that crosslinking increases when a crosslinking agent with a high functionality is introduced into the hard segment, which interferes with hydrogen bonding between the N–H and C=O groups.
XRD analysis was performed to study the crystallinity of the SMPU samples. The patterns are shown in Fig. 2c, and the pristine PCL-diol pattern is shown in Fig. S2. PCL-diol showed a strong crystal peak at 2θ = 21.3°, whereas the XRD patterns of the SMPU samples showed a broad diffraction peak at 2θ = 15–30°, which represents the semi-crystalline properties of SMPU; this suggests that the crosslinked bonds were formed between the hard and soft segments after CO, COM, or COT was introduced as a crosslinking agent. This bond formation limited the mobility required for the soft segment to form a crystal structure. In particular, the full width at half maximum (FWHM) of the broad peak was in the order of CO < COM < COT, which confirms that the crosslinking density increased with increasing functionality of the polyols used, resulting in the formation of a more amorphous structure.
Thermal properties of SMPUs
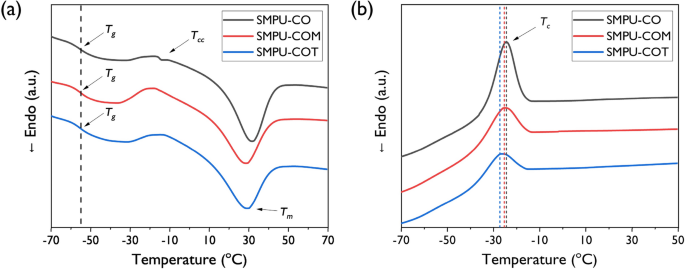
The thermal properties of SMPU-CO, SMPU-COM, and SMPU-COT were analyzed through DSC and the 2nd heating scan are shown in Fig. 3a, and values for melting temperature (Tm), transition temperature (Tg), degree of crystallinity (Xc), and others are listed in Table 2. The peaks for Tg, crystallization temperature (Tc), and melting temperature (Tm) were observed in all SMPU samples. It is known that Tg does not appear in PCL-diol, but Tg became detectable after PCL-diol was incorporated into the crosslinked PU structure. However, almost no change in Tg was observed upon varying the introduced crosslinking agent. It was found in other studies that Tg increased due to inter-domain mixing between the soft and hard segments when small molecule triols such as trimethylolpropane were used as a crosslinking agent. In the cases of CO, COM, and COT, this distinction could not be made because of their very bulky structures. The heat of fusion per gram of PCL-diol in the PU of SMPU-CO, SMPU-COM, and SMPU-COT decreased to 43.9, 34.4, and 32.2 J/g, respectively, as shown in Table 2. The PCL-diol soft-segment crystallinities were 32.4, 25.4, and 23.0%, respectively, which followed the order of SMPU-CO > SMPU-COM > SMPU-COT. Low heat of fusion and low crystallinity indicate the presence of a poorly separated phase. In our study, the crosslinking density increased as the functionality of the crosslinking agent increased, which limited phase separation and thereby decreased crystallinity; this was consistent with the previously reported XRD results. Meanwhile, in addition to glass transition and melting transition, exothermic transition was observed in the heating and cooling scans; this transition is known to be caused by the recrystallization behavior of the PCL soft segment. As shown in Fig. 3b, in the cooling scan, the melt crystallization temperatures were − 24, − 25, and − 27 °C for SMPU-CO, SMPU-COM, and SMPU-COT, respectively; a tendency to gradually shift to a lower temperature was observed. Moreover, the crystal enthalpy was found to gradually decrease to 22.9, 15.3, and 12.7 J/g, respectively, which can be attributed to the disruption of rapid crystal formation upon the increase in the functionality of the crosslinking agent. Meanwhile, in the DSC scans of all SMPUs, the glass transition and melting transition of the hard segment were not observed.
Thermal stability of SMPUs
To verify the thermal stability of the synthesized SMPU samples, TGA was performed under a nitrogen atmosphere. TG thermograms and their derivative thermogravimetry (DTG) curves are depicted in Fig. 4, and thermal stability parameters are listed in Table 3. When 5% of SMPU-CO, SMPU-COM, and SMPU-COT decomposed, the temperatures were 332, 336, and 348 °C, respectively, and those for 95% decomposition gradually increased to 538, 540, and 549 °C, respectively. As shown in Fig. 4b, the thermal decomposition process of the SMPU samples proceeded in three stages, which agrees with previous PU research results28,29. Table 3 shows the pyrolysis temperatures at each stage measured from the DTG curves. In the first stage, the decomposition of urethane bonds, which impart relatively low thermal stability, began to occur. During the first stage of thermal degradation, SMPU-COT decomposed at the lowest temperature of 385 °C and SMPU-CO at the highest temperature of 403 °C. During the second stage, the oligomerization of the triglyceride structure of the vegetable oil occurred. In the case of SMPU-CO, SMPU-COM, and SMPU-COT, the maximum decomposition temperatures at the second stage were 467, 496, and 501 °C, respectively, which increased significantly with functionality of crosslinkers. This suggested that more thermal energy was required to break the structure of highly branched crosslinking networks. The last stage represents the final decomposition of the remaining materials left after the second stage of thermal degradation; at this stage, SMPU-COT showed the highest decomposition temperature at 562 °C. Based on the TGA results, it was confirmed that the thermal stability of PUs was greatly improved after the introduction of high-functionality crosslinking agents30,31.
Mechanical properties
Figure 5 shows the stress–strain curves and graphs for tensile strength and tensile elongation of the synthesized SMPU samples. All samples had a smooth transition without a yield point in the elastic and plastic deformation regions. As expected, the tensile strength was increased as the crosslinking density increased, but the tensile elongation tended to decrease. SMPU-CO showed a tensile strength of 12.8 MPa and a tensile elongation of 1138%. In comparison, SMPU-COT showed a tensile strength of 23 MPa and a tensile elongation of 840%. The hard segment in the segmented PU acted as a reinforcement. In elastic materials, the tensile strength is inversely proportional to the distance between the crosslinking points32. Therefore, the number of crosslinking points increased after the crosslinking agents having high functionalities was introduced, which resulted in a considerable increase in tensile strength.
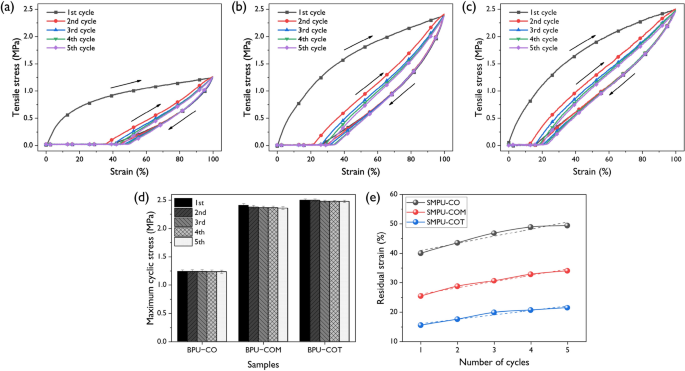
To investigate the elastic recovery characteristics of the synthesized SMPUs, five cyclic mechanical tests were performed; the results are shown in Fig. 6. There was a large hysteresis loop between the 1st and 2nd cycles in all SMPU samples. However, no significant difference was observed from the 2nd to 5th cycles. During the 1st cycle, strong softening occurred due to the orientation of the soft segment chain and the rearrangement of the hard segment. This ‘trained’ structure exhibited reversible behavior unless the initial extension was exceeded in subsequent cycles. Figure 6d shows a graph of the change in cyclic stresses of the SMPU samples. In the cases of SMPU-COM and SMPU-COT, the cyclic stress decreased until the 3rd cycle and then remained stable. Strain recovery characteristics are also shown in Fig. 6e. In the 1st cycle, the residual strains of SMPU-CO, SMPU-COM, and SMPU-COT were 40.0, 25.4, and 15.5%, which increased as the crosslinking density increased. Soft segment deformation occurred mainly during the 1st conditioning cycle, which was mostly reversible, while hard segment deformation occurred at the defect in the PU, which was irreversible. Therefore, higher contents of the hard segment and a greater number of crosslinking points led to more prominent elastic recovery characteristics25. Moreover, if the initial elongation were not exceeded in subsequent cycles, no further deformation of the hard segment occurred and, thus, high elastic recovery could be maintained.
The stress–strain profiles of elastic recoveries at 100% for the (a) SMPU-CO, (b) SMPU-COM, and (c) SMPU-COT under 5 constant cycles at room temperature. (d) The changes in maximum cyclic stresses for the SMPUs according to recovery cycles with different crosslinkers. (e) Residual strain of the SMPU-CO, SMPU-COM, and SMPU-COT.
Shape memory properties
In order to evaluate the shape memory characteristics, shape memory test using UTM was performed with the following procedures: The sample is first heated above the transition temperature. The original length of the sample is L0. The melting temperature and crystallization temperature of the soft segment of the prepared SMPUs were considered as the transition temperatures. After sufficient time was given, the sample was stretched to 200% and 400%. The stretched length of sample is nL0 (n = 2 and 4). Then, the specimen is cooled below its transition temperature, followed by removing tensile load. When the temperature was raised above the transition temperature again, shape recovery of the specimen occurred. A detailed description is shown in Fig. S3. The shape recovered length of sample is L2. Shape fixity (Rf) and shape recovery ratios (Rr) are calculated by using follow equations:
$$R_{f} = \frac{{\left( {L_{1} – L_{0} } \right)}}{{\left( {2L_{0} – L_{0} } \right)}} = \frac{{\left( {L_{1} – L_{0} } \right)}}{{L_{0} }}$$
(2)
$$R_{r} = \frac{{\left( {L_{1} – L_{2} } \right)}}{{\left( {L_{1} – L_{0} } \right)}}$$
(3)
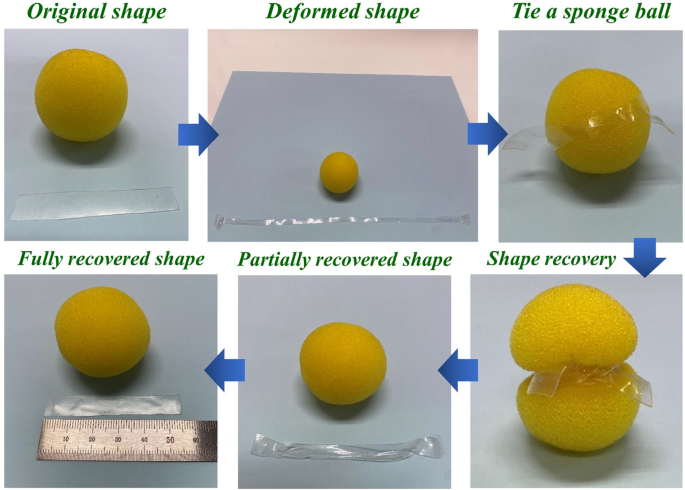
Typical shape-memory testing profile of SMPU-COT at strains of 200 and 400% are shown in Fig. 7a and b and the results are summarized in Fig. 7c and d. The shape-memory tests were carried out in three consecutive cycles. In the case of 200% shape memory test, the shape fixity values were 98.4, 98.9, and 99.0% in the first, second and third cycles, respectively. Generally, shape memory properties are believed to be related to covalent network points in polymers and physical entanglement. It is noted that the hard domains originating from the crosslink of the SMPUs minimize the permanent deformation caused by the creeping of the polymer chain. This is crucial for SMPUs to provide high shape memory retention. It was confirmed that better shape fixity and recovery characteristics were obtained as the functionality of the crosslinker increased. Shape memory characteristics of SMPU-COT was demonstrated and recorded using a digital camera (Fig. 8). First, the SMPU-COT film was deformed in the longitudinal direction at a temperature above the transition temperature and cooled down to fix the deformed shape. A sponge ball was tied once with the modified the SMPU-COT film. When heated to a temperature above the transition temperature again, the SMPU-COT film tightened the sponge ball tightly. The SMPU-COT film was removed from the sponge ball by untying the knot. And heating up to the transition temperature continuously, the shape returned to the original shape.